EPIDYOLEX® is the only regulatory approved prescription CBD anti-seizure medication, which means that it has a safety and efficacy profile that has been thoroughly evaluated in clinical trials. It also means that this plant-based, never synthetic, highly purified form of CBD is manufactured to meet the high standards of Good Manufacturing Practices and the World Health Organization's Good Agricultural Practices.
WHAT IS EPIDYOLEX® (CBD)?
CBD is one of the main cannabinoids present in the Cannabis sativa plant and can often be confused with the other main cannabinoid in cannabis, tetrahydrocannabinol (THC). Despite their similarities, these two cannabinoids have very different effects. THC is primarily responsible for the euphoric effects of cannabis, whereas CBD has been associated with anticonvulsant and neuroprotective effects.12

HOW EPIDYOLEX® WORKS
- Structurally distinct from other ASMs3
- Reduces neuronal hyperexcitability through multiple mechanisms of action1
- Does not exert its anticonvulsant effects through interaction with cannabinoid receptors1
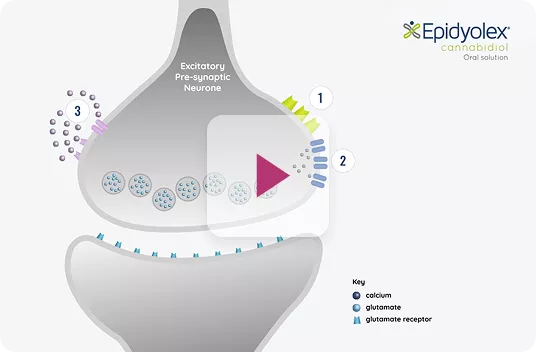
Reduction of neuronal hyperexcitability via 3 main actions (theoretical):1,11
EPIDYOLEX® HAS AN ALTERNATIVE MECHANISM OF ACTION
That provides a different way to treat seizures vs. conventional ASMs 1,11
NO EUPHORIC EFFECTS
The euphoric effects of cannabis are mediated through cannabinoid type 1 (CB1) receptors which are mostly located in the brain. Whilst CBD and THC are structurally very similar, they work via different mechanisms in the body. CBD has limited CB1 binding ability and therefore no euphoric effects.12
HOW IS EPIDYOLEX® PRODUCED?
EPIDYOLEX® is produced in the UK at our industry-leading growing and manufacturing facilities.
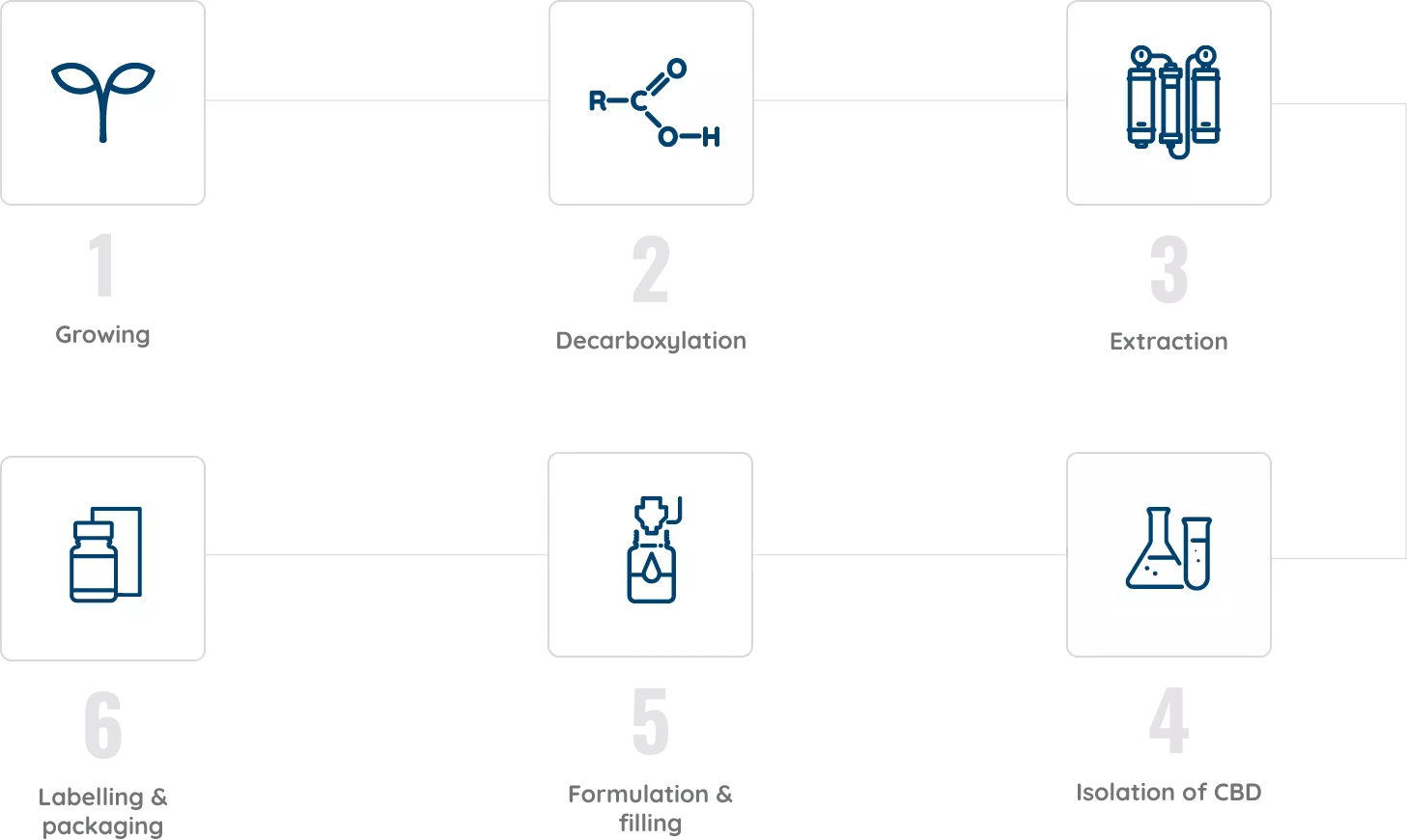
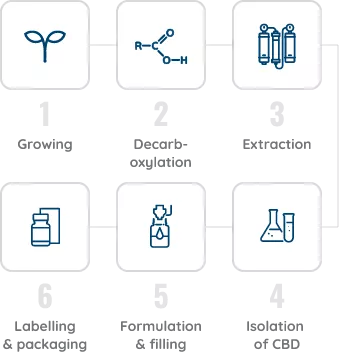
Jazz Pharmaceuticals control the entire process start-to-end, from selectively breeding and growing all plants according to Good Agricultural Practice, to extraction and purification according to GxP standards, through to accurate product labelling and secure shipping. This ensures batch-to-batch consistency for all patients, so you can prescribe with confidence.
The 6 stages of production
ABBREVIATIONS
ASM: anti-seizure medication; CB1: Cannabinoid Receptor 1; CBD: cannabidiol; DS: Dravet syndrome; GxP: Good Practice; LGS: Lennox-Gastaut syndrome; THC: tetrahydrocannabinol; TSC: tuberous sclerosis complex.
* Clobazam currently does not have marketing authorisation in Norway.
† The Decision Forum Nye Metoder has determined that treatment with EPIDYOLEX® must be initiated by a neurologist or paediatrician, who has experience in treating patients with LGS, DS or TSC.
- EPIDYOLEX® Summary of Product Characteristics. Approved: May 2023.
- Raga S, et al. Epileptic Disord. 2021;23(1):40–52.
- Marchese F, et al. SN Compr Clin Med. 2021;3:2167–2179.
- Patel AD, et al. Epilepsia. 2021;62(9):2228–2239.
- Scheffer IE, et al. Epilepsia. 2021;62(10):2505–2517.
- Thiele EA, et al. Epilepsia. 2022;63(2):426–439.
- Berg A, et al. Epilepsy Research. 2023;107280:0920-1211.
- Privitera M, et al. Epilepsia. 2021;62:1130–1140.
- Cohen JM, et al. Epilepsia. 2021;62:2218–2227.
- Thiele EA, et al. Epilepsia. 2022;63(2):426–439.
- Gray, RA and Whalley, BJ. Epileptic Disorders. 2020;(S1)22:S10–S15.
- Billakota S, et al. Curr Opin Neurol. 2019;32(2):220–226.
REFERENCES
- EPIDYOLEX® Summary of Product Characteristics. Approved: May 2023.
- Raga S, et al. Epileptic Disord. 2021;23(1):40–52.
- Marchese F, et al. SN Compr Clin Med. 2021;3:2167–2179.
- Patel AD, et al. Epilepsia. 2021;62(9):2228–2239.
- Scheffer IE, et al. Epilepsia. 2021;62(10):2505–2517.
- Thiele EA, et al. Epilepsia. 2022;63(2):426–439.
- Berg A, et al. Epilepsy Research. 2023;107280:0920-1211.
- Privitera M, et al. Epilepsia. 2021;62:1130–1140.
- Cohen JM, et al. Epilepsia. 2021;62:2218–2227.
- Thiele EA, et al. Epilepsia. 2022;63(2):426–439.
- Gray, RA and Whalley, BJ. Epileptic Disorders. 2020;(S1)22:S10–S15.
- Billakota S, et al. Curr Opin Neurol. 2019;32(2):220–226.
ABBREVIATIONS
ASM: anti-seizure medication; CB1: Cannabinoid Receptor 1; CBD: cannabidiol; DS: Dravet syndrome; GxP: Good Practice; LGS: Lennox-Gastaut syndrome; THC: tetrahydrocannabinol; TSC: tuberous sclerosis complex.
FOOTNOTES
* Clobazam currently does not have marketing authorisation in Norway.
† The Decision Forum Nye Metoder has determined that treatment with EPIDYOLEX® must be initiated by a neurologist or paediatrician, who has experience in treating patients with LGS, DS or TSC.