DOSING

Recommended dosing schedule1
| LGS | DS | TSC | |
|---|---|---|---|
| First week | Starting dose 5 mg/kg/day (2.5 mg/kg taken twice daily) | ||
| Second week | Maintenance dose 10 mg/kg/day (5 mg/kg taken twice daily) | 10 mg/kg/day (5 mg/kg taken twice daily) | |
| Further titration as applicable (incremental steps) | Weekly increments 5 mg/kg/day (2.5 mg/kg administered twice daily) | ||
| Maximal recommended dose | 20 mg/kg/day (10 mg/kg taken twice daily) | 25 mg/kg/day (12.5 mg/kg taken twice daily) | |
Based on individual clinical response and tolerability, dosage titrations should be increased in weekly increments of 2.5 mg/kg administered twice daily (5 mg/kg/day). Any dose increases above 10 mg/kg/day, to maximum recommended dose, should be made considering individual benefit and risk and with adherence to the full monitoring schedule.1
Discontinuation
If EPIDYOLEX® has to be discontinued, the dose should be decreased gradually. In clinical trials cannabidiol discontinuation was achieved by reducing the dose by approximately 10% per day for 10 days. A slower or faster down-titration may be required, as clinically indicated, at the discretion of the prescriber.1
Response optimisation
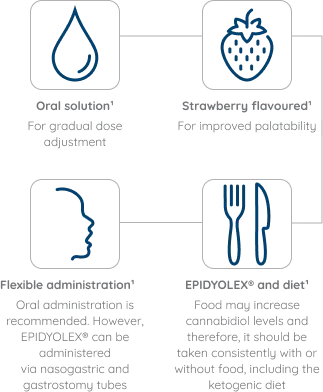
EPIDYOLEX® can be co‑administered with other commonly prescribed ASMs and medicines, with/without dose adjustment1
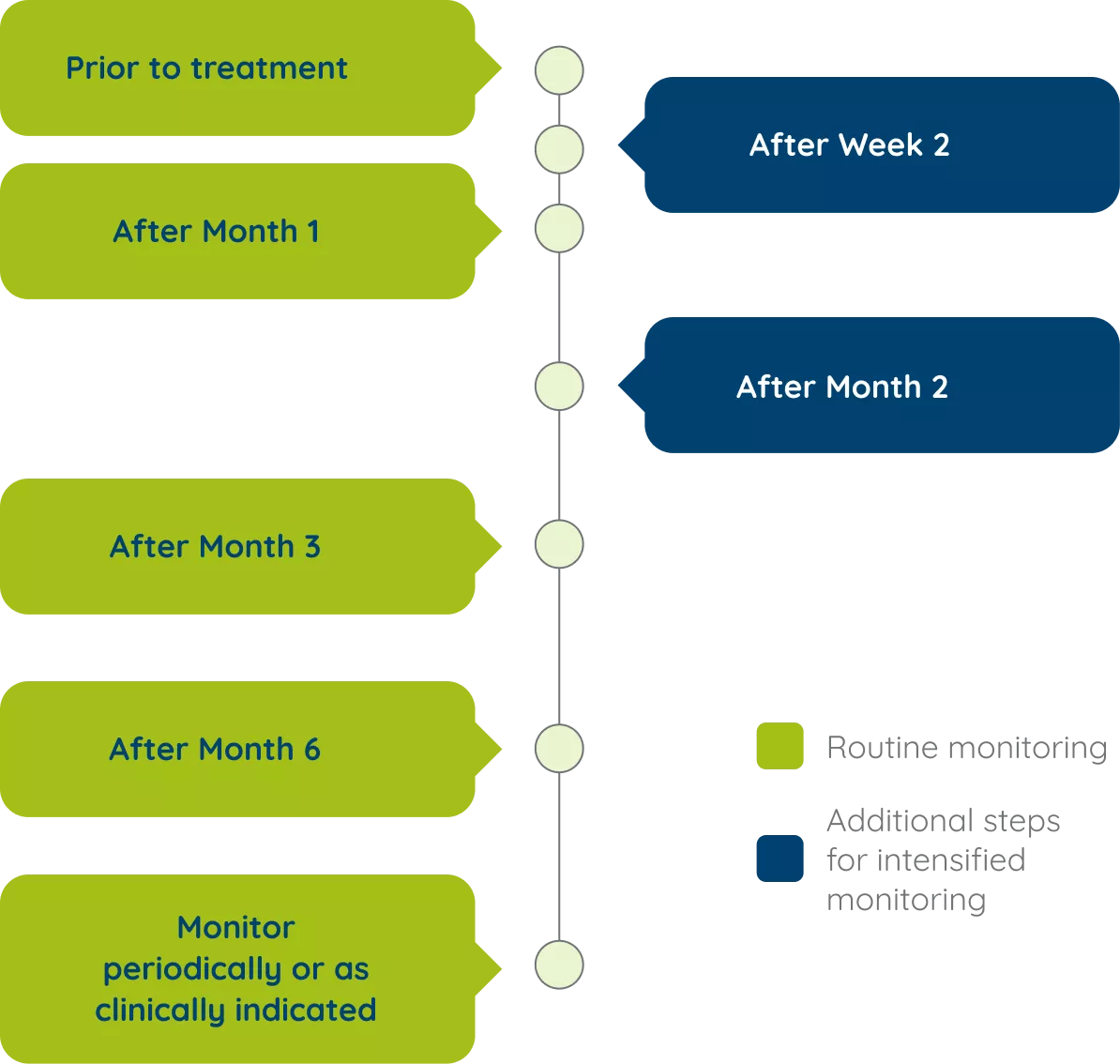
Monitoring schedule for patients on EPIDYOLEX®1
EPIDYOLEX® dosing should be adjusted in special populations1
REFUSJON AV LGS, DS og TSC
EPIDYOLEX® er innført av Beslutningsforum i 2022 (LGS og DS) og i 2023 (TSC) og finansieres av sykehus via H-resept for alle tre indikasjoner:
- Adjuvant behandling av anfall i forbindelse med Lennox-Gastaut syndrom (LGS) eller Dravets syndrom (DS), gitt sammen med klobazam, hos pasienter som er 2 år eller eldre. https://www.nyemetoder.no/metoder/cannabidiol-epidyolex/
- Adjuvant behandling av anfall i forbindelse med tuberøs sklerose-kompleks (TSC) hos pasienter som er 2 år og eldre. https://www.nyemetoder.no/metoder/cannabidiol-epidyolex-indikasjon-ii/
SAFETY
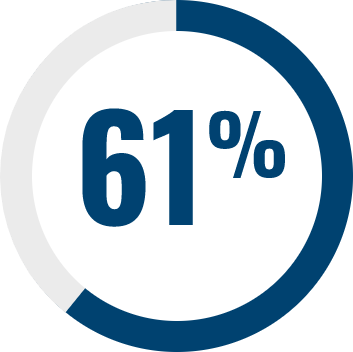
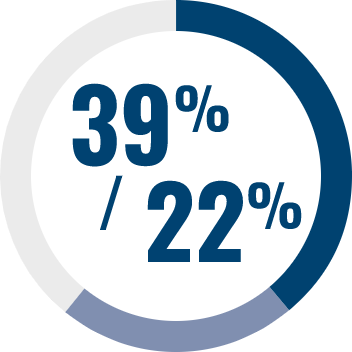
Adverse events generally occur early in treatment and are consistent across all indications11–14
The most common adverse events (≥10% of patients) associated with EPIDYOLEX® are:1
- Decreased appetite
- Somnolence
- Vomiting
- Pyrexia
- Diarrhoea
- Fatigue
Long-term tolerability of EPIDYOLEX® in open-label extension has been demonstrated as consistent with results from clinical trials in LGS, DS and TSC1,4–6
ABBREVIATIONS
ALT: alanine transaminase; ASM: anti-seizure medication; AST: aspartate transaminase; CBD: cannabidiol; DS: Dravet syndrome; LGS: Lennox-Gastaut syndrome; OLE: open-label extension; TSC: tuberous sclerosis complex; ULN: upper limit of normal.
* Patients were exposed to EPIDYOLEX® as part of a large clinical trial programme within LGS, DS and TSC.1
# Clobazam currently does not have marketing authorisation in Norway.
- Epidyolex® Summary of Product Characteristics. Approved: May 2023.
- Raga S, et al. Epileptic Disord. 2021;23(1):40–52.
- Marchese F, et al. SN Compr Clin Med. 2021;3:2167–2179.
- Patel AD, et al. Epilepsia. 2021;62(9):2228–2239.
- Scheffer IE, et al. Epilepsia. 2021;62(10):2505–2517.
- Thiele EA, et al. Epilepsia. 2022;63(2):426–439.
- Privitera M, et al. Epilepsia. 2021;62:1130–1140.
- Cohen JM, et al. Epilepsia. 2021;62:2218–2227.
- Berg A, et al. Epilepsy Research. 2023;107280:0920–1211.
- Thiele EA, et al. JAMA Neurol. 2021;78(3):285–292.
- Privitera M, et al. Epilepsia. 2021;62(5):1130–1140.
- Cohen JM, et al. Epilepsia. 2021;62(9):2218–2227.
- Wu JY, et al. Epilepsia. 2022;63(5):1189–1199.
- Gunning B, et al. Acta Neurol Scand. 2021;143:154–163.
- Nabbout R, et al. Epileptic Discord. 2020;22(S1):23–28.
REFERENCES
- Epidyolex® Summary of Product Characteristics. Approved: May 2023.
- Raga S, et al. Epileptic Disord. 2021;23(1):40–52.
- Marchese F, et al. SN Compr Clin Med. 2021;3:2167–2179.
- Patel AD, et al. Epilepsia. 2021;62(9):2228–2239.
- Scheffer IE, et al. Epilepsia. 2021;62(10):2505–2517.
- Thiele EA, et al. Epilepsia. 2022;63(2):426–439.
- Privitera M, et al. Epilepsia. 2021;62:1130–1140.
- Cohen JM, et al. Epilepsia. 2021;62:2218–2227.
- Berg A, et al. Epilepsy Research. 2023;107280:0920–1211.
- Thiele EA, et al. JAMA Neurol. 2021;78(3):285–292.
- Privitera M, et al. Epilepsia. 2021;62(5):1130–1140.
- Cohen JM, et al. Epilepsia. 2021;62(9):2218–2227.
- Wu JY, et al. Epilepsia. 2022;63(5):1189–1199.
- Gunning B, et al. Acta Neurol Scand. 2021;143:154–163.
- Nabbout R, et al. Epileptic Discord. 2020;22(S1):23–28.
ABBREVIATIONS
ALT: alanine transaminase; ASM: anti-seizure medication; AST: aspartate transaminase; CBD: cannabidiol; DS: Dravet syndrome; LGS: Lennox-Gastaut syndrome; OLE: open-label extension; TSC: tuberous sclerosis complex; ULN: upper limit of normal.
FOOTNOTES
* Patients were exposed to EPIDYOLEX® as part of a large clinical trial programme within LGS, DS and TSC.1
# Clobazam currently does not have marketing authorisation in Norway.