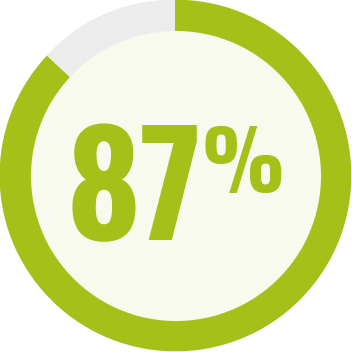
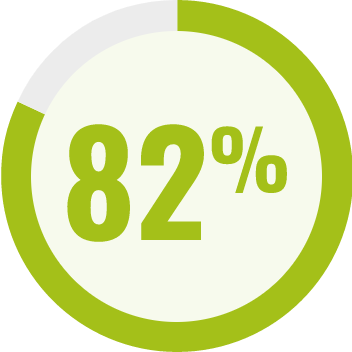
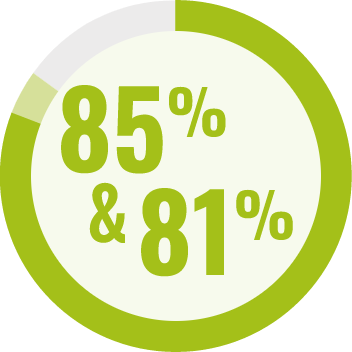
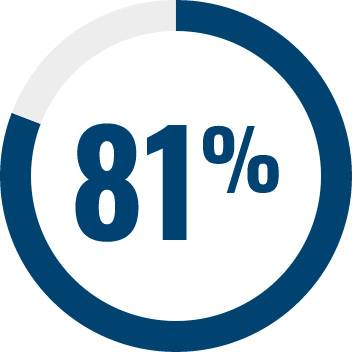
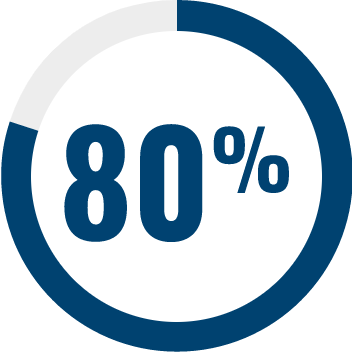
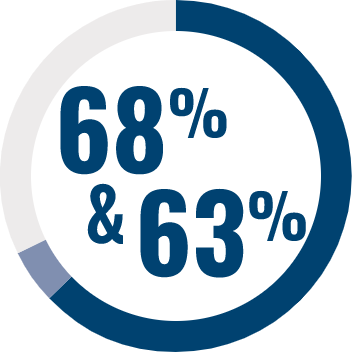
RESPONDENTS REPORTED IMPROVEMENT IN NON-SEIZURE RELATED OUTCOMES:1
RESPONDENTS REPORTED IMPROVEMENT IN NON-SEIZURE RELATED OUTCOMES:1
BECOME is a global outcomes survey of caregivers of 498 people with LGS or DS that assessed changes in BEhavior, COgnition, and More with EPIDYOLEX® after ≥3 months of treatment, compared to the month prior to initiation1#
ABBREVIATIONS
CLB: Clobazam; DS: Dravet syndrome; LGS: Lennox-Gastaut syndrome.
* Clobazam currently does not have marketing authorisation in Norway.
# Survey contained only LGS and DS patients with 55% of paediatric patients and 40% of adult patients also taking CLB.*1 Results were similar across paediatric and adult patients.1
- Berg A, et al. Epilepsy Research. 2023;107280:0920-1211.
REFERENCE
- Berg A, et al. Epilepsy Research. 2023;107280:0920-1211.
ABBREVIATIONS
CLB: Clobazam; DS: Dravet syndrome; LGS: Lennox-Gastaut syndrome.
FOOTNOTES
* Clobazam currently does not have marketing authorisation in Norway.
# Survey contained only LGS and DS patients with 55% of paediatric patients and 40% of adult patients also taking CLB.*1 Results were similar across paediatric and adult patients.1