EPIDYOLEX® DEMONSTRATED A CLINICALLY SIGNIFICANT REDUCTION IN CONVULSIVE SEIZURES## VS. PLACEBO IN DS PATIENTS1
Reduction in convulsive seizure frequency vs. placebo can be achieved as early as the second week of EPIDYOLEX® treatment (nominal p-value=0.03†)9
Primary endpoint: Reduction from baseline in monthly convulsive seizure## frequency1

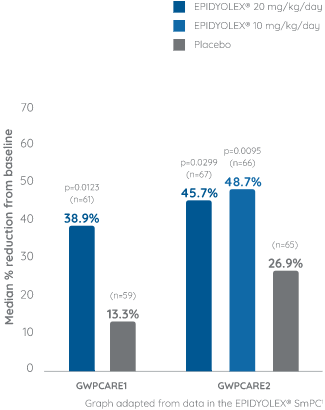
Approximately 65% of the patients were taking concomitant clobazam*. Of the patients that were not taking clobazam*, the majority had previously taken and subsequently discontinued clobazam* treatment.
Primary endpoint: Reduction from baseline in monthly convulsive seizure## frequency1

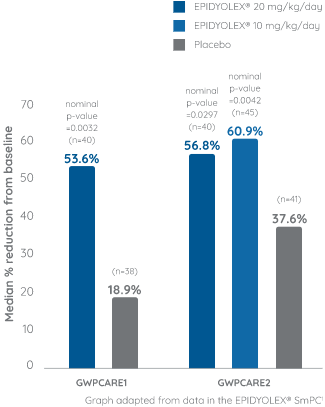
Approximately 65% of the patients were taking concomitant clobazam*. Of the patients that were not taking clobazam*, the majority had previously taken and subsequently discontinued clobazam* treatment.
DS PATIENTS TREATED WITH EPIDYOLEX® BENEFITTED FROM MORE CONVULSIVE DROP SEIZURE##-FREE DAYS PER MONTH VS. PLACEBO1
In a post-hoc analysis
+1.3 – 2.2
Convulsive drop seizure##-free days per month1
EPIDYOLEX® 20 mg/kg/day
+2.7
Convulsive drop seizure##-free days per month1
EPIDYOLEX® 10 mg/kg/day
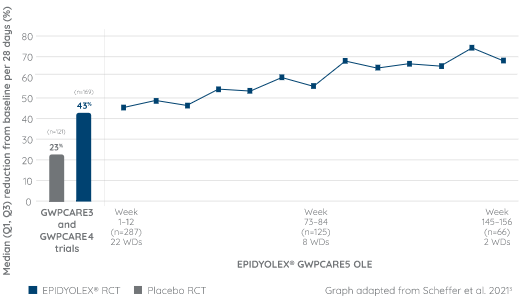
SUSTAINED SEIZURE REDUCTION VS. BASELINE WAS MAINTAINED OVER 3 YEARS5
High retention rate in OLE: 55% of patients with DS completed 3 years of treatment5
- The mean modal dose of EPIDYOLEX® during the OLE trial was 22 mg/kg/day3
- The maximum recommended dose in DS is 10 mg/kg twice daily (20 mg/kg/day)1

EPIDYOLEX® has been assessed in 5 Phase III clinical trials within Lennox-Gastaut syndrome (LGS), Dravet syndrome (DS) and Tuberous Sclerosis Complex (TSC) including 938 patients and 3 open-label extension studies.1,4
ABBREVIATIONS
CLB: Clobazam; DS: Dravet syndrome; GIC: Global Impression of Change; LGS: Lennox-Gastaut syndrome; OLE: open-label extension; RCT: randomised controlled trial; TSC: tuberous sclerosis complex.
* Clobazam currently does not have marketing authorisation in Norway.
† Drop seizures were defined as atonic, tonic, or tonic-clonic seizures that led or could have led to a fall or injury.1
“ A month was defined as 28 days. No statistical analysis was performed for these data.
§ Analysis performed using overall population, in which 54.4% of patients were receiving concomitant CLB* treatment.4
## Convulsive seizures were defined as atonic, clonic, and tonic-clonic seizures.1
†† Assessed in overall population, where 64.4% of patients were receiving concomitant CLB*. p-values reported vs. placebo.
§§ Analysis performed using overall population, in which 68% of patients were receiving concomitant CLB* treatment.5
### TSC-associated seizures included: focal motor seizures with or without impairment of awareness, focal seizures evolving to bilateral motor seizures & generalised seizures (tonic-clonic, tonic, clonic, or atonic).
** The overall condition improved by at least one category on the seven-category Caregiver GIC scale. Improvements were based on measures of GIC scores at the last visit.10
- EPIDYOLEX® Summary of Product Characteristics. Approved: May 2023.
- Raga S, et al. Epileptic Disord. 2021;23(1):40–52.
- Marchese F, et al. SN Compr Clin Med. 2021;3:2167–2179.
- Patel AD, et al. Epilepsia. 2021;62(9):2228–2239.
- Scheffer IE, et al. Epilepsia. 2021;62(10):2505–2517.
- Thiele EA, et al. Epilepsia. 2022;63(2):426–439.
- Berg A, et al. Epilepsy Research. 2023;107280:0920-1211.
- Privitera M, et al. Epilepsia. 2021;62:1130–1140.
- Cohen JM, et al. Epilepsia. 2021;62:2218–2227.
- Thiele EA, et al. Epilepsia. 2022;63(2):426–439.
- Gray, RA and Whalley, BJ. Epileptic Disorders. 2020;(S1)22:S10–S15.
- Billakota S, et al. Curr Opin Neurol. 2019;32(2):220–226.
- Wu JY, et al. Epilepsia. 2022;63(5):1189–1199.
REFERENCES
- EPIDYOLEX® Summary of Product Characteristics. Approved: May 2023.
- Raga S, et al. Epileptic Disord. 2021;23(1):40–52.
- Marchese F, et al. SN Compr Clin Med. 2021;3:2167–2179.
- Patel AD, et al. Epilepsia. 2021;62(9):2228–2239.
- Scheffer IE, et al. Epilepsia. 2021;62(10):2505–2517.
- Thiele EA, et al. Epilepsia. 2022;63(2):426–439.
- Berg A, et al. Epilepsy Research. 2023;107280:0920-1211.
- Privitera M, et al. Epilepsia. 2021;62:1130–1140.
- Cohen JM, et al. Epilepsia. 2021;62:2218–2227.
- Thiele EA, et al. Epilepsia. 2022;63(2):426–439.
- Gray, RA and Whalley, BJ. Epileptic Disorders. 2020;(S1)22:S10–S15.
- Billakota S, et al. Curr Opin Neurol. 2019;32(2):220–226.
- Wu JY, et al. Epilepsia. 2022;63(5):1189–1199.
ABBREVIATIONS
CLB: Clobazam; DS: Dravet syndrome; GIC: Global Impression of Change; LGS: Lennox-Gastaut syndrome; OLE: open-label extension; RCT: randomised controlled trial; TSC: tuberous sclerosis complex.
FOOTNOTES
* Clobazam currently does not have marketing authorisation in Norway.
† Drop seizures were defined as atonic, tonic, or tonic-clonic seizures that led or could have led to a fall or injury.1
“ A month was defined as 28 days. No statistical analysis was performed for these data.
§ Analysis performed using overall population, in which 54.4% of patients were receiving concomitant CLB* treatment.4
## Convulsive seizures were defined as atonic, clonic, and tonic-clonic seizures.1
†† Assessed in overall population, where 64.4% of patients were receiving concomitant CLB*. p-values reported vs. placebo.
§§ Analysis performed using overall population, in which 68% of patients were receiving concomitant CLB* treatment.5
### TSC-associated seizures included: focal motor seizures with or without impairment of awareness, focal seizures evolving to bilateral motor seizures & generalised seizures (tonic-clonic, tonic, clonic, or atonic).
** The overall condition improved by at least one category on the seven-category Caregiver GIC scale. Improvements were based on measures of GIC scores at the last visit.10